Health
How can the development cycle of medical device prototypes be shortened by 60% while controlling costs

Biotech component and medical device development is challenged by the regulatory approval process, cost, and length of the product development cycle. Issues are found in the late stages of product development, resulting in product delays and cost overruns. The basic challenge is the unvalidated stages of product development, starting from functionality and material compatibility.
In this article, the proposed solution is the strategic development of rapid prototypes using the correct materials and processes for complete product verification before large-scale production. The next sections will examine the five key factors for applicability of the approach in the medical domain.
How to Choose the Most Suitable Rapid Prototyping Technology for Medical Device Development?
The selection of the proper types of rapid prototyping technology is the key first step in successful validation. It is mainly driven by the target purpose of the specific type of rapid prototype device, whether visualization, functional testing (e.g., fluid path integrity), fit and assembly, or initial biocompatibility testing.
For highly detailed and smooth surface aesthetic components as well as the creation of transparent components, Stereolithography or SLA would be the first choice. In scenarios where robust and complex-shaped functional components are needed without the necessity of having support structures, the Selective Laser Sintering or SLS process would be most beneficial. Yet, when the examination of metal surgical instruments or the validation under genuine material properties is required, the use of prototype CNC machining would be imperative due to its capacity to process isotropic materials.
Agencies such as the American FDA consider the early stages of prototyping to be very important, deeming it an advantageous step in the process of reducing risks in both clinical and post-market performances. An effective rapid prototyping process entails understanding verification objectives and assigning suitable technology, material, and technique based on those objectives. This is very helpful in achieving success in both Medical Device Manufacturing and Biotechnology Component Production.

What are the Key Factors of Biocompatibility of Rapid Prototyping Materials?
In medical terms, materials for rapid prototyping materials involve more than form and functionality, as they have a direct relation with patient safety and regulatory issues. Hence, knowing what these important factors involve can be critical for anyone involved in such projects.
The Three Pillars of Material Selection
The selection criteria for prototype materials for the use in the medical sector has to meet three fundamental standards:
- Biocompatibility:
The major gateway in this category is biocompatibility. Materials should be tested according to standards such as ISO 10993 to be non-cytotoxic, non-sensitizing, and non-irritating when they come into contact with the human body. Companies offering biomedical prototyping services should provide material choices compatible with biocompatibility standards of material testing. - Sterilizability:
The prototype needs to be able to withstand their intended method of sterilization, such as autoclaving, gamma radiation, and Ethylene Oxide, without degrading their properties and being representative of the end product. - Mechanical Performance:
The strength, toughness, and wear resistance of the material being used should be able to satisfy the requirements of simulated real-world usage, or else the functional testing would be rendered meaningless.
Phased Implementation of Material Usage
A strategic approach to material choice must be employed at different stages of prototyping:
- Early-Stage Conceptual Validation
The skills required during this stage involve understanding the material properties and performing assembly. Making rapid prototyping parts using general-purpose engineering plastics, such as ABS-like materials, is cost-effective and quick.
- Mid to Late Stage Functional and Compliance Validation
With an evolving design, it would be better to use materials which are closer to the end application. For example, a biopolymer like medical-grade Nylon (PA12) can be preferred for a Long-Lasting Enclosure, whereas a trial implant or a high-load instrument exam may require a titanium alloy (Ti6Al4V) and CNC Machining, or even a stainless steel material. A Rapid Prototyping Cost-Benefit Guide can help make the right decisions.
How Does an Efficient Rapid Prototyping Process Optimize the Medical Device Development Cycle?
An optimized and efficient rapid prototyping process is the engine that drives the compression of development schedules. It is “much more than the process of simply printing or machining a part” and is, in fact, a systematic process of engineering.
A optimized process would begin with professional 3D data preparation and Design for Manufacturability, where problems are analyzed and solved before they develop. This would be followed by accurate technology and material choice, depending on the objectives for the verification. In the Rapid Manufacturing process, whether it is done by 3D printing/CNC Machining Healthcare components, it would be important to have high-precision machines and attention to detail. Final processing, including cleaning and sterilization, and Quality Inspection would be the last hurdle before the prototypes are considered reliable.
For instance, developing prototypes for manufacturability analysis with CNC Machining Healthcare instrument prototypes can help detect potential issues such as thin walls, corners, and difficult-to-manufacture features that can save weeks and months of mold changes later on. This whole procedure also acts as excellent evidence for complying with ‘design verification’ criteria for quality management systems, such as the ISO 13485 standard. Associating with rapid prototyping services companies that have certifications such as ISO 9001, ISO 13485, and AS9100D can help guarantee all aspects related to the procedures above.
Why is Prototype CNC Machining Important in Surgical Instrument Production?
The process of Surgical Instrument Manufacturing requires high accuracy, robustness, and biocompatibility. In such a case, the involvement of prototype CNC machining is extremely crucial.
Its highest advantage is the ability to create functional rapid prototypes with exactly the same materials as the final product-for example, Stainless Steel 316L, Titanium alloys. This means that, other than being geometrically accurate, the prototype will be functionally representative, as it maintains the same isotropic mechanical properties and response to heat treatment, including microstructure, as the production part will have. It gives the possibility to engineers to perform the most realistic fatigue tests, torque tests, repeated actuation tests, and rigorous sterilization cycle validations with data having high confidence for final design decisions.
On the other hand, most metal 3D printing technologies may have their own limits such as suffering from interlayer bonding, higher surface roughness, and internal porosity in making these kinds of high-stress instrument prototypes. In such complicated instruments with very tiny geometries, for example, articulated joints or internal channels, 5-axis CNC machining can produce high precision monolithic fabrication to ensure assembly accuracy and functional integrity, which is critical to validating complex device designs.
How to Evaluate the Comprehensive Value of Rapid Prototyping Services Beyond Unit Price?
For Biotechnology Component Production, which has high value and involves strict regulations, it would be unwise to simply compare the cost per service for rapid prototyping services. A thorough evaluation for its overall value should instead extend to covering more extensive fields, such as its usefulness in enabling the attainment of the lowest possible cost and shortest possible time-to-market for the compliant product.
Key Activities Unrelated to the Quote
Firstly, the benefit of a specialty service provider is seen in its engineering support services, namely the capability to ensure in-depth DFM feedback in order to eradicate errors prior to occurrence. Second, the importance of having a quality control function is seen in the fact that a provider that maintains high levels of quality standards is one that maintains strong process controls.
A Systematic Evaluation Framework
There would be benefit in creating a multi-dimensional evaluation structure including consideration of the following factors:
- Technical Match: Does the supplier provide the complete range of necessary processes (additive, subtractive, molding processes)?
- Quality and Compliance Certifications: Does it have essential quality certifications, such as ISO 13485? Are cleanroom facilities available?
- Industry Experience and Case Studies: Are there successful projects in similar fields of medicine or biotech?
- Overall Delivery Capabilities: Does it deliver the whole process, right from communications and design help to post-processing and documentation, smoothly and effectively?
Realizing Lowest Total Cost
For projects which have stringent technical requirements, it is important to select an appropriate partner who can end-to-end meet requirements. An appropriate professional service in this aspect is the Medical Device Rapid prototyping service which can be best exemplified through JS Precision. It is evident that the actual worth is established when it reduces the number of cycles with an end objective of minimizing project cost and time to market.
Conclusion
In conclusion, a successful medical devices or biotechnology solution is significantly reliant on an early adoption of a rapid prototyping solution with a structured, scientific approach. Each phase of a project, whether technology or material selection, significantly influences a solution’s effectiveness and speed to market, with authorities viewing rapid prototyping approaches as a necessary pathway rather than an optional tool, especially from an innovation viewpoint from key players in this particular sector or market.
In case you are experiencing issues with creating prototypes for healthcare and biotech-related projects, and you are looking for expert help that is fast, accurate, and compliant with standards, you are welcome to get in touch with our team for a free consultation.
Author Bio
The article has been contributed by a professional who has over a decade of experience in the field of precision manufacturing, specifically in the domain of offering unique and different rapid prototyping and customized manufacturing services to the medical and biotech sectors.
FAQs
Q1: What is the typical lead time for rapid prototype manufacturing?
A1: The lead times vary significantly based on technology and complexity. From 24-48 hours for simple parts-the SLA is a good example-ranges to 3-7 working days for complex functional metal prototypes via CNC machining. Vacuum casting will take 5-10 days for small batches.
Q2: What specific standards do the rapid prototypes of the medical device have to adhere to?
A2: The main ones would involve standards for biocompatibility, such as ISO 10993; sterilizability may also be necessary, and a manufacturing cleanroom. Prototype materials and production processes should allow for final regulatory submission requirements.
Q3: How does one choose between 3D printing and CNC machining for medical prototypes?
A3: 3D printing works for complex geometries and one-time-use surgical guides. CNC machining is preferred when the prototype requires the exact mechanical properties of the final metal material, especially for surgical instruments.
Q4: How is the confidentiality of medical prototype design data protected?
A4: Suppliers should be selected on the basis of their stringent data security measures, usually implemented through Non-Disclosure Agreements (NDAs), secure file transfer protocols, or data management practices.
Q5: Can rapid prototypes be directly used in clinical tests?
A5: This is material-, process-, and regulatory-dependent, although there are approved materials and processes for additive manufacturing that have been qualified for the actual implant, although this is not the case for the prototypes.

 Blog8 months ago
Blog8 months ago[PPT] Human Reproduction Class 12 Notes
- Blog8 months ago
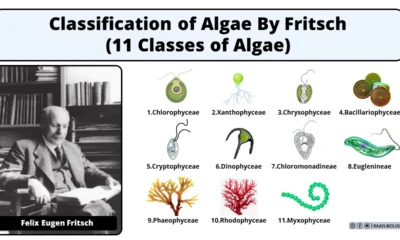
Contribution of Indian Phycologists (4 Famous Algologist)
- Blog8 months ago
PG TRB Botany Study Material PDF Free Download

 Blog8 months ago
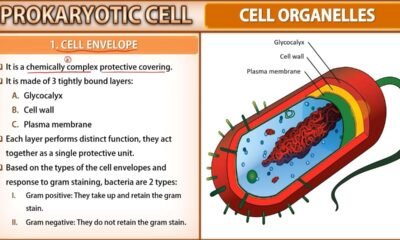
Blog8 months agoCell The Unit of Life Complete Notes | Class 11 & NEET Free Notes

 Blog8 months ago
Blog8 months ago[PPT] The living world Class 11 Notes

 Blog8 months ago
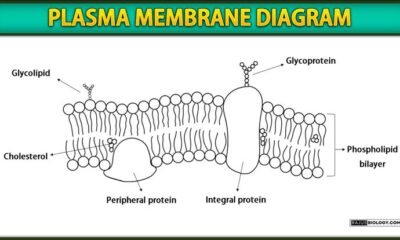
Blog8 months agoPlasma Membrane Structure and Functions | Free Biology Notes

 Blog8 months ago
Blog8 months agoJulus General Characteristics | Free Biology Notes

 Blog8 months ago
Blog8 months agoClassification of Algae By Fritsch (11 Classes of Algae)