Blog
Methods of Gene Transfer Short Notes

Method # 1 Calcium Chloride (CaCl2) Mediated DNA Transfer
Principle:
In the process of transformation all bacterial cells cannot uptake the exogenous DNA molecule. Those who are capable to take are called competent cells.
So our aim in this step is to make bacterial cells more competent so that the possibility of transferring of the recombinant DNA into the host cell increases to a higher fold. CaCl2 makes the cell wall of the bacteria more permeable to the exogenous DNA and thus increases the competence of the host cell.
Procedure:
Growing E. Coli cells are isolated and suspended in 50 mM CaCl2 at a concentration of 108-1010 cells/ml. The cells may be incubated for 12- 24 hr. to increase the frequency of transformation. The recombinant DNA is then added.
Efficient transformation takes only a few minutes and the cells are plated on a suitable medium for the selection of transformed clones. The frequency of transformed cells is 106-107 per mg of plasmid DNA; this is about one transformation per 10,000 plasmid molecules.
The transformed cells are suitably diluted and spread thinly on a suitable medium so that each cell is well separated and produces a separate colony.
Generally, the medium is so designed that it permits only the transformed cells to divide and produce colonies. This frequency can be further improved by using special E. Coli strains, e.g., SK1590, SK1592, X1766, etc.
Method # 2 Electroporation
Electroporation or electro-permeabilization is the process of applying electrical field to a living cell for a brief duration of time in order to create microscopic pores in the plasma membrane called electro-pores.
This technique is used for transferring the recombinant DNA molecule into wide range of hosts starting from bacteria to plant (plant protoplasts) and animal cells.
Principle:
The phospholipid molecules of the plasma membrane are not static. When we apply electric field to them their kinetic energy increases resulting in the increase in the membrane permeability at certain points.
This is exactly where we see the formation of electro-pores. The recombinant DNA can pass through these transient pores before they close.
Procedure:
In this process cells are mixed with the recombinant DNA and the mixture is placed in a small chamber with electrodes connected to a specialized power supply. Then a brief electric impulse is discharged across the electrodes, which makes pores (holes) in the plasma membrane.
These pores remain for some time and are again resealed themselves. Recombinant DNA enters the cell which are removed and plated in fresh selective medium. The process of selection is then applied to isolate cells carrying recombinant DNA.
Method # 3 Liposome Encapsulation (Lipofection)
This technique is found very successful in the transfection of plant protoplasts and animal host cells.
Principle:
Liposomes are microscopic vesicles developed in a laboratory environment. Each liposome is a spherical ball like structure made up of phospholipid bilayers with a hollow central space, allowing liposomes to interact directly with cells.
A liposome can fuse with the cell membrane of the taken host cell and can deliver its content to it. The recombinant DNA enclosed in the liposome vesicles penetrates into the protoplast of the host cell.
Procedure:
In this technique the recombinant DNA, which is negatively charged at a near neutral pH because of its phosphodiester backbone, is mixed with the lipid molecules with positively charged (cationic) head groups.
The lipid molecules form a bilayer around the recombinant DNA molecules. This results in the formation of liposomes which are further mixed with the host cells.
Most eukaryotic cells are negatively charged at their surface, so the positively charged liposomes interact with the cells. Cells take up the lipid-recombinant DNA complexes, and some of the transfected DNA enters the nucleus.
Method # 4 Microinjection
This is the direct introduction of the recombinant DNA into the host cell. This technique has been used successfully with both plant and animal cells. In this procedure the cell is held on a glass capillary by gentle suction.
The microinjection needle is made by drawing out a heated glass capillary to a fine point. Using a micromanipulator (a mechanical device for fine control of the capillary) the needle has been inserted into the nucleus of the host cell.
One obvious disadvantage is that this technique is labour-intensive and not suitable for primary cloning procedures where large numbers of recombinants are required.
However, in certain specialised cases it is an excellent method for targeting DNA delivery once a suitable recombinant has been identified and developed to the point where microinjection is feasible.
Method # 5 Biolistic Particle Delivery System
A gene gun or a biolistic particle delivery system is a device which can directly bombard small particles coated with the recombinant DNA on the nucleus of the target cell. This technique is often simply referred to as bio-ballistics or biolistics and has been successfully used in the transfection of both plant and animal cells.
In this technique the recombinant DNA is coated with microscopic tungsten particles known as micro-projectiles, which are then accelerated on a macro-projectile by firing a gunpowder charge or by using compressed gas to drive the macro-projectile.
At one end of the ‘gun’ there is a small aperture that stops the macro-projectile but allows the micro-projectiles to pass through. When directed at cells, these micro-projectiles carry the DNA into the cell and, in some cases, stable transformation will occur.
Method # 6 Protoplast Fusion
This technique is used for introducing gene of interest into plant and animal cells. In this technique first we transfer the recombinant DNA into a bacterial cell then dissolve its cell wall by treating it with lysozyme.
After this we fuse the host protoplast with the bacterial cell (lacking cell wall) by the help of polyethylene glycol (PEG). The transfected cells are then selected by suitable methods.
Method # 7 Virus Mediated Gene Transfer
In other way the gene can be packed into a virus and allow it to infect the host cell without harming it in any way. This method can be used both for the transformation of prokaryotic host cell as well as transfection of eukaryotic host cells.
In the case of bacterial host cells the recombinant DNA can be packed into the empty head of a specially designed bacteriophage (e.g., lambda phage) and allow the virion to infect the host cell.
Similarly, while transfecting the plant host cells we can follow the similar strategy by using plant viruses like Caulimo virus and Gemini virus. In the case of animals, retrovirus infection of embryos has been used for the production of transgenic mice.
This virus has been found to be an efficient vector system for animals. The virus carrying the gene of interest transfers it into the genome of embryonic cells leading to its integration and production of transgenic animals.

 Blog8 months ago
Blog8 months ago[PPT] Human Reproduction Class 12 Notes
- Blog8 months ago
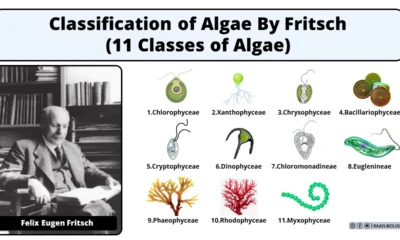
Contribution of Indian Phycologists (4 Famous Algologist)
- Blog8 months ago
PG TRB Botany Study Material PDF Free Download

 Blog8 months ago
Blog8 months agoCell The Unit of Life Complete Notes | Class 11 & NEET Free Notes

 Blog8 months ago
Blog8 months ago[PPT] The living world Class 11 Notes

 Blog8 months ago
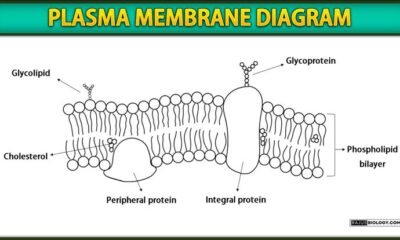
Blog8 months agoPlasma Membrane Structure and Functions | Free Biology Notes

 Blog8 months ago
Blog8 months agoJulus General Characteristics | Free Biology Notes

 Blog8 months ago
Blog8 months agoClassification of Algae By Fritsch (11 Classes of Algae)